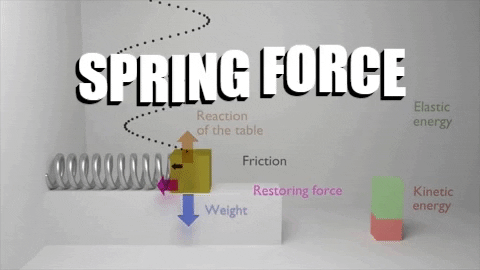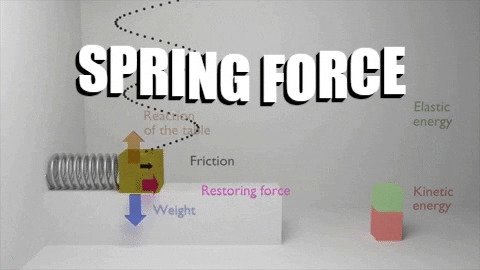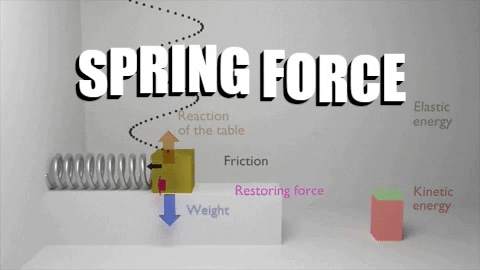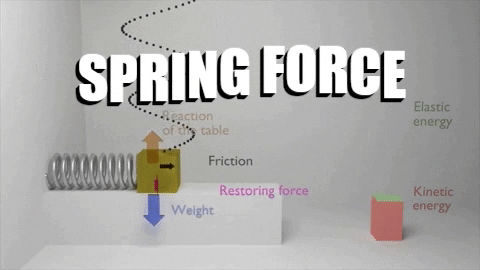
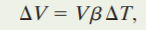The first law of thermodynamics stated that:
"The total energy of an isolated system is constant;energy can be transformed from one form to another, but can be created nor destroyed"
3 basic terms needed in thermodynamics
1.thermodynamics system - is everything inside the boundaries including the space
2. Sorroundings of a system-is everything of that system.The sum of the system and the sorroundings is the universe.
3.Boundary of a system- is the real or imaginary surface which separates that system from its sorroundings.
3 basic terms needed in thermodynamics
1.thermodynamics system - is everything inside the boundaries including the space
2. Sorroundings of a system-is everything of that system.The sum of the system and the sorroundings is the universe.
3.Boundary of a system- is the real or imaginary surface which separates that system from its sorroundings.
Types of System in thermodynamics
1.Closed System- type of system where only energy can cross the system boundaries
2.Open System - is a type of system where both mass and energy can cross the system boundaries
3.Isolated System-type of system where both mass and energy cannot cross the system boundaries.
 |
| (Image 1 )Bread inside the oven |
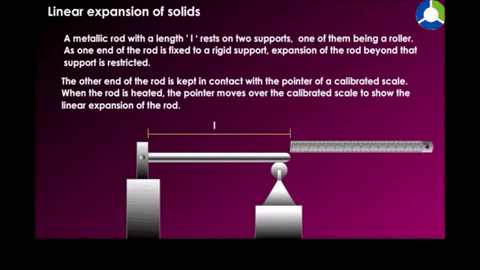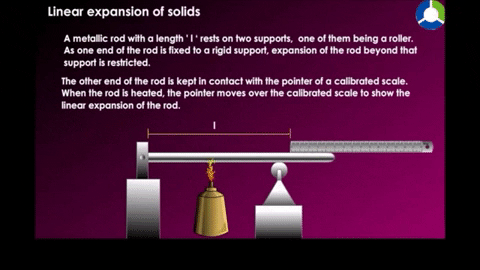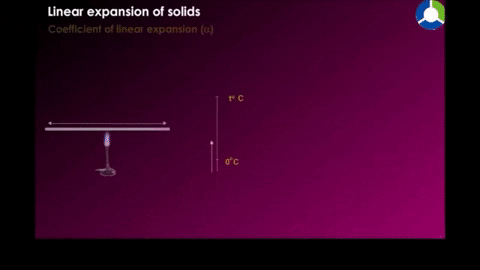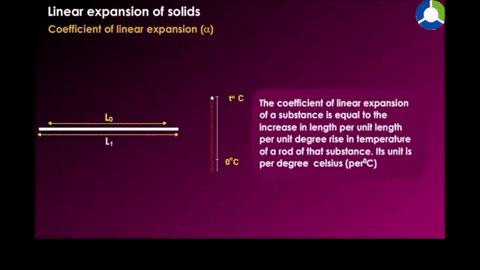
Image 1 shows us an example of closed system where the system is whats inside the oven(bread) including the space. When heat is transfer or added into the system there is the change in internal energy due to rise in temperature,an increase in temperature or a change in state.
Internal energy is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system as a whole due to external force fields.
Formula for internal energy:
ΔU =Q – W
where Q is the heat flowing in or out the system ,W is the work done by or onto the system and U is the internal energy.
Sign convention
- When heat is supplied to the system, it increases the internal energe, so Q is taken as positive(Q > 0)
- Work was done on the system also increases the internal energy, so it is also taken as positive. (W > 0),In this case, the first law of thermodynamics is written as:
ΔEint=Q +W
- When heat is rejected by the system,it decreases the internal energy, so it is taken as negative. (Q <0)
- Work done by the system decreases the internal energy, so it is taken as negative (W <0)
Example of first law of thermodynamics
One example of first law of thermodynamics is the diesiel engine When an engine burns fuel it converts the energy stored in the fuel's chemical bonds into useful mechanical work and into heat.
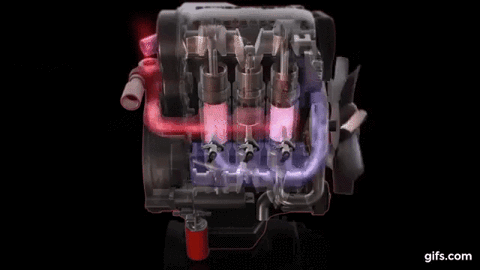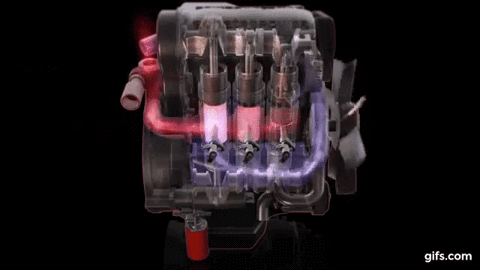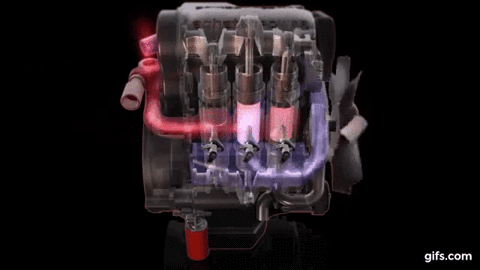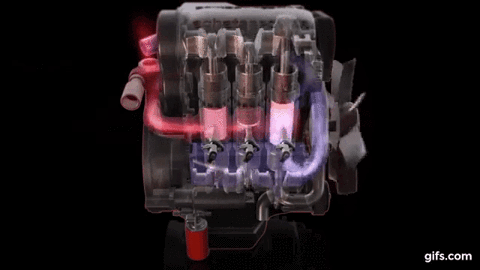
The conservation of energy principle defined by the first
law of thermodynamics says that when all of the fuel's energy is released by
burning in the engine's cylinders it doesn't disappear. The total quantity of
energy stays the same and must be accounted for. In the case of the diesel
engine shown below it either becomes thermal energy (heat) or mechanical energy
(work). For every 100 units of fuel energy that is burned in the engine a
hundred units of converted energy has to end up somewhere. It doesn't disappear
1.)Adiabatic process - it is a process which no heat can transfer in or out the system. There is no transfer in heat so Q=0
ΔEint=Q +W
since
Q = 0 ,SO
ΔEint = W
2.) Isovolumetric Process-A process where volume is held constant, since volume remains constant, the work done will be zero W=0.
ΔEint =Q +W
Since W=0,SO
⇒ Q = ΔEint
3.)Isothermal process-A process in which temperature held constant, Since the temperature remains constant in this process so the internal energy also must remain constant :
ΔEint=Q +W
0 = Q + W
⇒ Q =-W
4.) Isobaric process- A process in which pressure held constant.

where:

and
,
Problem examples:
1.Assume that there is no change in internal energy while the system is doing work of 20J .Compute for the heat added or taken away by the system.
Our formula for internal energy is
ΔEint=Q -W(Negative because the system is the one doing work)
given that there is no change in internal energy (ΔEint =0) while the system is doing work of 20J (W=20J).Subtituting it to our formula we get:
0=Q-20
Q=20J
The heat added to our system is 20J when the system did 20J of work.
2.A bottled water with the heat of 5J is placed on a tracking field exposed by a sun giving more 20J of heat ,Compute for the work done by or onto the bottled water if the bottled water has the change in internal energy of 40J.
Our formula for Work is
W = ΔEint - Q
given 20J plus 5J of heat and 40J of change in internal energy we get:
W = 40J-(20+5)
W=15J
The work in the system is 15J.
3. A balloon having a gas initial volume of 3L .The balloon transfers 300J of heat to the sorroundings this makes the gas final volume 1.7L. Compute for the internal energy given our external pressure 1x105
Formula for internal energy given pressure and volume is :
ΔEint=−PΔV+ Q
Given pressure 100000 Pa ,Transfer of heat 300J and change in volume from 3L to 1.7L Subtituting these to the equation we get:
ΔEint= -(100000Pa)(3L-1.7L) - 300J = -130300J
Watch video for broad and clear explanation of First law of thermodynamics and internal energy:
watch the video of me Explaining the summary of First law of thermodynamics
Kindly answer this quiz to see how much you learn from this site:
References: